
The chief use of chromium is to form alloys with iron, nickel, or cobalt. It is an ingredient in several important catalysts. More than half the production of chromium goes into metallic products, and about another third is used in refractories. The Cr-Cr bond distance in a range of these quadruply bonded species has been found to vary between 195-255 pm. Each Cr(II) ion has 4 d electrons but the complex is found to be diamagnetic which is explained by the formation of a quadruple bond between the two metal ions.

2H 2O is an example of a Cr(II) complex which is reasonably stable in air once isolated.
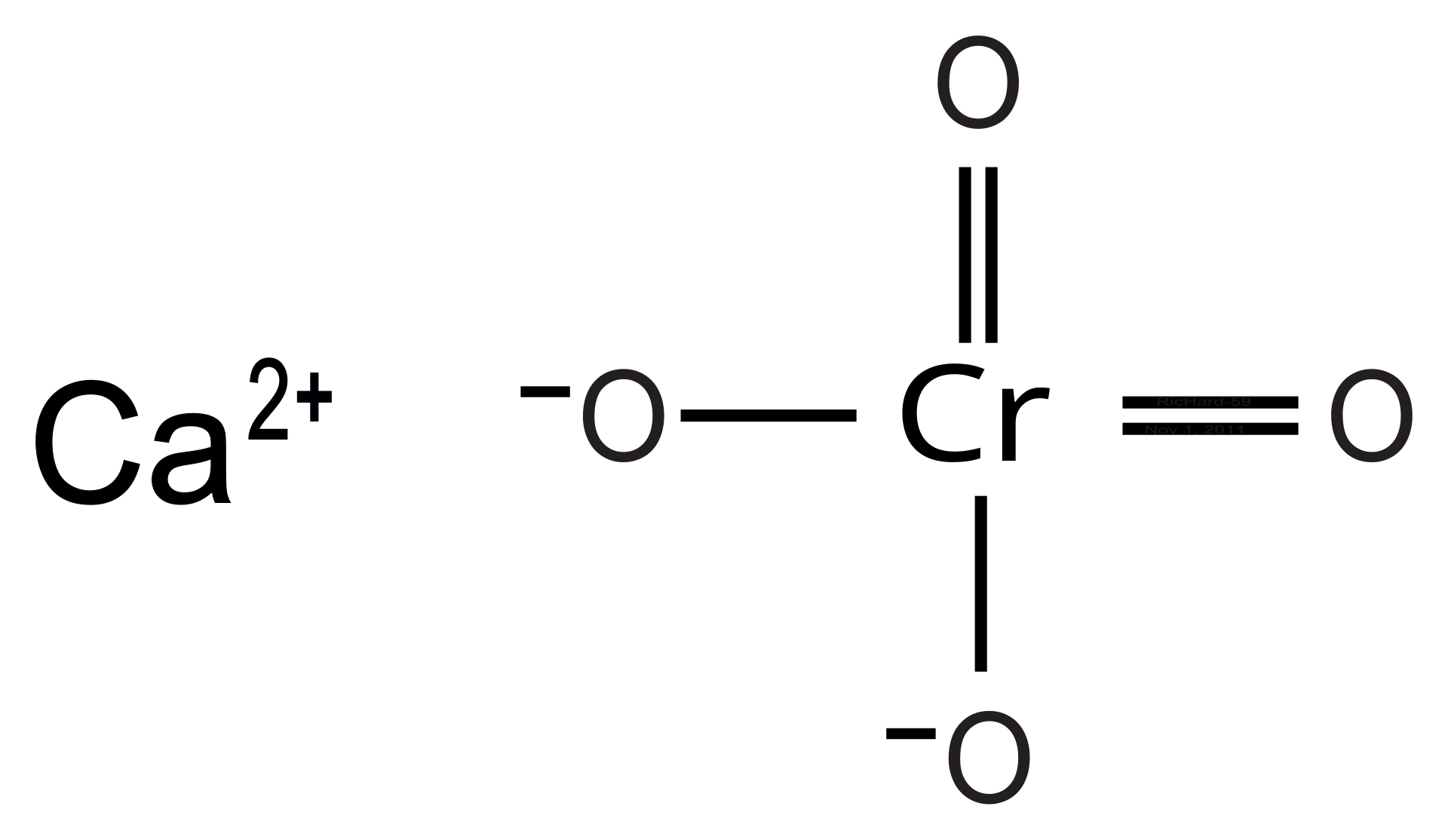
#Formula for chromium chloride manual#
See the laboratory manual for this course for a range of other Cr(III) complexes for which you should know the structure. This allows a small amount of the Cr(II) ion to be formed, which is very labile but unstable with respect to oxidation back to Cr(III). the dark green trans-Cl.2H 2O salt shown above, etc.Īnhydrous CrCl 3 reacts with pyridine only in the presence of Zinc powder.Hydrated chromium chloride, "CrCl 3.6H 2O", exists as hydrate isomers, including: Further deprotonation and polymerization can occur and, as the pH is raised, the final product is hydrated chromium(III) oxide or "chromic hydroxide".Īnhydrous CrCl 3 and hydrated "CrCl 3.6H 2O", The ease with which the proton is removed can be judged by the fact that the hexaaquo ion (pKa ~ 4) is almost as strong as acetic acid. This is thought to occur by the loss of a proton from coordinated water, followed by coordination of the OH - to a second cation: One of the most obvious characteristics of Cr(III) is that it is acidic i.e it has a tendency to hydrolyse and form polynuclear complexes containing OH - bridges in a process known as OLATION. pH 2-6 HCrO 4 - and Cr 2O 7 2- orange-red.World production of chromite ores approached 12 million tonnes in 1995.
The sodium chromate produced in the isolation of chromium is itself the basis for the manufacture of all industrially important chromium chemicals. Alternatively, the Cr 2O 3 can be dissolved in sulfuric acid to give the electrolyte used to produce the ubiquitous chromium-plating which is at once both protective and decorative. The main use of the chromium metal so produced is in the production of nonferrous alloys, the use of pure chromium being limited because of its low ductility at ordinary temperatures. Should be converted to less soluble forms and sent to disposal facilities. In closed bottles, air-tight for the anhydrous form. While less toxic than Cr(VI) compounds, chromium chloride is less toxic, although it's still quite harmful.

Reacting chlorine gas with hot chromium metal will also give anhydrous CrCl 3. Alternatively, hexahydrate chromium chloride is treated with thionyl chloride, yielding very dry CrCl 3.Ī more energetic route involves the carbothermic chlorination of chromium(III) oxide between 650–800 ☌.
hydrochloric acid.Ĭhromium chloride anhydrous can be prepared by reacting chromium hydroxide, chromium(III) oxide or just chromium metal with hydrochloric acid in methanol. Chromium(III) chloride will react with bases to give chromium hydroxide.Ĭhromium(III) chloride is a purple (anhydrous) or dark green (hydrated) solid.Ĭhromium chloride is sold by chemical suppliers.Ĭhromium chloride hexahydrate can be obtained by reacting chromium oxide, hydroxide or plain chromium metal with conc.


 0 kommentar(er)
0 kommentar(er)
